Product name:3-aminocyclohex-2-enone
MF:C6H9NO
Cas No.:5220-49-5
MW:111.14
Structure: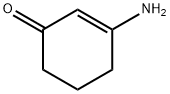
Purity:95%
Appearance:yellow solid
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
ammonium acetate; benzene;
Reactants are commercially availlable.
Into a 500 mL round bottom flask fitted with a Dean-Stark trap and a magnetic stirrer was added 150 mL of anhydrous benzene, 1,3-cyclohexanedione (5.6 g, 0.05 mol), and ammonium acetate (7.7 g, 0.1 mo l). The mixture was stirred continuously, and refluxed for 5 h. The reaction was allowed to reach room temperature. The precipitate was recrystallized with ethyl acetate to afford yellow crystals (1.7 3 g, 31percent yield). Mp-128-131 °C [lit. 128-131 °C (35)]. 1H NMR Acetonitrile-d3) delta -1.8-2.4 (6H, m, cyclohexene ring), 5.1 (1H, s, CH). 5.3-5.5 (2H, s, br, NH2).
