Product name:2-Bromo-5-methylthiazole
MF:C4H4BrNS
Cas No.:41731-23-1
MW:178.05
Structure: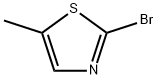
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
tert.-butylnitrite; acetonitrile;
Reactants are commercially availlable.
Imamura, Kenichiro, US2004/97425, A1, (2004) Preparation 30 2-Bromo-5-methyl-1,3-thiazole To a solution of 2-amino-5-methyl-1,3-thiazole (11.7 g) in acetonitrile (200 ml) was added dropwise tert-butyl nitrite (8.33 ml) at 0°C. followed by addit ion of copper(II) bromide (5 g) over 0.5 minutes. After stirring at 0°C. for 3 hours, the mixture w as concentrated and partitioned between 1N hydrochloric acid and ethyl acetate. The organic layer wa s washed with water, saturated aqueous sodium hydrogencarbonate solution and brine, dried over anhydrous magnesium sulfate, filtered through a pad of Celite, and concentrated in vacuo to give the titl e compound (3.24 g) as an oil. ESI-MS: 177.8(M+H) 1H-NMR (300 MHz, CDCl3) delta 7.25(s, 1H), 2.44(s, 3H).
