Product name:Ethyl 4-iodo-5-methylisoxazole-3-carboxylate
MF:C7H8INO3
MW: 281.05
Cas No.:1356600-24-2
Structure: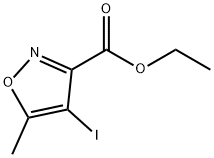
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthetic route is as follows:
N-iodo-succinimide; trifluoroacetic acid; sodium hydrogencarbonate; water; ethyl acetate;
Reactants are commercially availlable.
RAO, D, Shivanageshwara, WO2012/11125, A1, (2012) Intermediate 49: Ethyl 4-iodo-5-methyl-1, 2-oxazole-3-carboxylateTo a 25 mL RB flask fitted with a magnetic stirrer was charged with 1 mL of TFA. To the stirred solvent were added ethyl 5-methyl-1 ,2- oxazole-3-carboxylate (0.1 g, 0.6 mmol) and N-iodosuccinimide (0.289 g, 1.2 mmol). After addition, t he reaction mixture was heated at 65 °C for 3 h. After completion of the reaction, the reaction mixt ure was diluted with ethyl acetate (10 mL), the organic layer was washed with saturated NaHC03 solution (10 mL), thiosulfate solution (10 mL), water (25 mL) and finally with brine solution (10 mL). Th e organic layer was dried over anhydrous Na2S04 and the solvent was removed under reduced pressure. The product was obtained as a brown liquid (0.12 g, yield: 66.2percent). 1H NMR (300 MHz, CDCI3): de lta 4.36-4.43(q, 2H), 2.49(s, 3H), 1.34-1.39(t, 3H).
