Product name:Methyl 2-broMo-3-forMylbenzoate
MF:C9H7BrO3
Cas No.:750585-94-5
MW:243.05
Structure: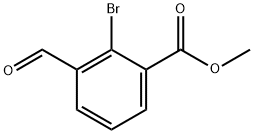
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
N-bromosuccinmide; dibenzoyl peroxide; dimethyl amine; tetrachloromethane; water;
Reactants are commercially availlable.
BENAROUS, Richard, WO2012/140243, A1, (2012) To a solution of methyl 2-bromo-3-methylbenzoate (28a) (3.35 g, 15.0 mmol) in carbon tetrachloride (35 mL) were added A/-bromosuccinimide (5.20 g, 29 mmol) and benzoyl peroxide (363 mg, 1 .5 mmol). The mixture was degassed for 15 minutes an d refluxed overnight. The mixture was filtered and the filtrate was concentrated in vacuo. The residue was dissolved in 40percent dimethylamine (46 mL) and irradiated (200W) for 15 minutes at 60°C. The solution was extracted with dichlorome thane (2x30 mL). The organic layers were dried over sodium sulfate and concentrated in vacuo to provide methyl 2-bromo-3-formylbenzoate (28b) (2.12 g, 8.72 mmol, 58percent) as a white solid.1 H NMR (400 MHz, CDCI3) 3.97 (s, 3H), 7.49 (t, J = 8.0 Hz, 1 H), 7.89 (dd, J = 1 .0 Hz, J= 8.0 Hz, 1 H), 8.00 (dd, J = 1 .0 Hz, J= 8.0 Hz, 1 H), 10.49 (s, 1 H).
