Product name:2-Amino-5-bromo-4-methylthiazole
MF:C4H5BrN2S
MW: 193.0649
Cas No.:3034-57-9
Structure: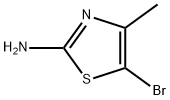
Purity:95%
Appearance:brown solid
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
bromine; sodium hydrogencarbonate; chloroform; water;
Reactants are commercially availlable.
Institut de Recherches Chimiques et Biologiques Appliquees (I.R.C.E.B.A.), US5322846, A1, (1994) Step 1: Synthesis of 2-amino-5-bromo-4-methylthiazole A solution of bromine in chloroform, consisting of 66.7 ml (1.30 mol) of Br2 in 1000 ml of CHCl3, is added dropwise to a solution of 120 g (1.05 m ol) of 2-amino-4-methylthiazole in 2300 ml of CHCl3, with stirring. A precipitate appears during the addition. Stirring is maintained for 48 h. The reaction medium is then filtered and the hydrobromid e is washed with methylene chloride and then with pentane. The hydrobromide is dissolved in 2000 ml of water and then rendered basic by the addition of 850 ml of a 10percent aqueous solution of sodium bicarbonate. This solution is then extracted with methylene chloride. The organic phase is dried over sodium sulfate. A crystalline residue is obtained after removal of the solvent under vacuum. Brow n crystals: m=155 g (crude yield: 76percent) M.p.KB =112°-113°C . 1 H NMR (delta ppm, DMSO) 2.05 (s, 3H, CH3); 7.15 (s, 2H, NH2).
