Product name:6-Benzyl-5,7-dihydro-5,7-dioxopyrrolo[3,4-b]pyridine
MF:C14H10N2O2
Cas No.:18184-75-3
MW:238.24
Structure: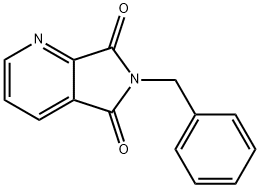
Purity:97%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
acetic anhydride; ethanol;
Reactants are commercially availlable.
Daiichi Pharmaceutical Co., Ltd., US5654318, A1, (1997) REFERENCE EXAMPLE 6 6-Benzyl-5,7-dihydro-5,7-dioxopyrrolo[3.4-b]pyridine To 100 g of 2,3-pyridinedicarboxylic acid was added dropwise 170 ml of acetic anhydride at room temperature, and the mixture wa s heated up to 110°C., and stirred for 4 hours. After completion of the reaction, the solvent was removed under reduced pressure. To a residue was added 200 ml of diethyI ether, and a precipitated c rystal was collected by filtration and washed with diethyl ether (100 ml*4) to yield 86 g of an acid anhydride compound. To the product was added dropwise 76 ml of benzylamine while cooling with ice, and the mixture was stirred at 180°C. for 30 minutes. To the reaction mixture was added dropwise 1 70 ml of acetic anhydride while cooling with ice, followed by stirring at 110°C. for 2 hours. Afte r completion of the reaction, the reaction mixture was allowed to cool, and 500 ml of ethanol was added thereto. The thus formed crystal was collected by filtration and washed with ethanol (100 ml*3) to yield 89.4 g of the titled compound. Melting point: 163°-165°C. (recrystallized from ethanol) 1 H-NMR (400 MHz, DMSO-d6) delta ppm: 4.28 (2H, s), 7.26-7.34 (5H, m), 7.80 (1H, dd, J=7.8, 5.4Hz), 8. 31 (1H, dd, J=7.8, 1.5Hz), 8.99 (1H, dd, J=5.4, 1.5Hz)
