Product name:4'-CHLOROACETANILIDE
MF:C8H8ClNO
MW: 169.61
Cas No.:539-03-7
Structure: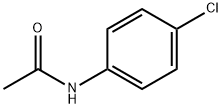
Purity:97%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthetic route is as follows:
bromine; dichloromethane; dichloromethane;
Reactants are commercially availlable.
Acetic acid (2 ml) was added to a stirred solution of 4-methylbenzenamine (0.54 g, 5 mmol) in CH2Cl2 . The solution was stirred for 30 min. After the solution cooled to 5 °C with ice bath, Br2 (2.00 g, 12.5 mmol) in 5 ml CH2Cl2 was slowly added to the stirred solution. The mixture was stirred for 5 h and neutralized by 10percent saturated aqueous sodium hydroxide solution. The mixture was extracted three times with 50 ml petroleum ether. The combined organic phase was dried over MgSO4, filtered, and the solvent was removed. The residue was purified by chromatography on silica gel with petroleum ether/ethyl ester (v/v = 15:1) to give 2,6-dibromo-4-methylbenzenamine (0.97 g, 73percent yield). 1H NMR (400 MHz, CDCl3): delta 2.20 (s, 3H, -CH3), 4.38 (s, 2H, -NH2), 7.19 (s, 2H, C6H2Br2CH3NH2). 1 3C NMR (400 MHz, CDCl3): delta 19.75 (-CH3), 108.69 (benzenamine carbon connected with Br), 129.31 (benzenamine carbon connected with CH3), 132.13 (benzenamine carbon), 139.48 (benzenamine carbon conn ected with NH2).
