Product name:2-Bromo-4-fomylthiazole
MF:C4H2BrNOSCas No.:5198-80-1MW:192.03Structure: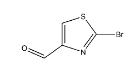
Purity:95%
Appearance:yellow solid
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
diisobutylaluminium hydride; hydrogenchloride; tetrahydrofuran; hexanes; dichloromethane; tetrahydrofuran; methanol; hexanes; dichloromethane; water;
Reactants are commercially availlable.
GLAXO GROUP LIMITED, WO2007/65669, A1, (2007) Ethyl 2-bromothiazole-4-carboxylate (3g, 12.7mmol) was taken up in THF and dichloromethane (100ml each) and cooled to -780C. 1 M DIBAL-H in hexanes (25.4ml, 25.4mmol) was added dropwise maintaining the temperature at <-70°C. The solution wa s stirred for 5h and quenched with MeOH (20ml). The solution was allowed to warm to room temperature, poured into 1 M HCI (200ml) and extracted with EtOAc (3x100ml). The combined organics were washed with brine (300ml), dried (Na2SO4) and c oncentrated. Purification by column chromatography on silica (0-50percent EtOAc\\40-60 pet. ether gradient) yielded the product as a white solid (0.862g). deltaH (CDCI3) 8.10(1 H, s), 9.95(1 H, s).
