Product name:5-BROMOTHIAZOLE-2-CARBOXYLIC ACID
MF:C4H2BrNO2S
Cas No.:957346-62-2
MW:208.03
Structure: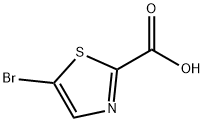
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
lithium diisopropyl amide; carbon tetrabromide; tetrahydrofuran; n-heptane; ethylbenzene; tetrahydrofuran; n-heptane; ethylbenzene;
Reactants are commercially availlable.
STEINHAGEN, Henning, WO2015/161928, A1, (2015) i) Thiazole-2-carboxylic acid (1.60 g, 12.4 mmol) was added to a solution of lithium diisopropylamin e (1 M in THF/heptane/ethylbenzene, 26 mmol, 26 mL) in dry THE (100 mL) at -78 °C and the RM was sti rred for 30 mi Tetrabromomethane (4.52 g, 13.6 mmol) was added and the RM was stirred for 2 h. The reactionRM was quenched by adding water (30 mL). The RM was allowed to reach RT and diluted by adding an aqueous saturated solution of NaHCO3 (50 mL). The RM was filtered through a pad of celite and extracted with EtOAc (50 mL). The organic layer was discarded and the aqueous layer acidified using a 1 N solution of HCI until pH 3-4. The solution was then extracted with EtOAc (3x 30 mL). The combined organic layer was dried over Na2SO4 and concentrated in vacuo to leave INT-18A (0.420 g, 2.02 mmol ,16percent). LCMS: caic. for [M+H]*=207.90, found 208.0.
