Product name:N-Formylpiperidine
MF:C6H11NO
MW:113.16
Cas No.:2591-86-8
Structure: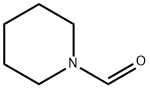
Purity:95%
Appearance:colorless liquid
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
C21H24N2; phenyl silane; tetrahydrofuran;
Reactants are commercially availlable.
Jacquet, Olivier, US2014/18576, A1, (2014) General procedure: The process for preparing formamide compounds of formula (I) may be carried out according to the following experimental protocol. Under an inert atmosphere in a glove box, the amine R1R2NH (1 molar equivalent), the (pre)c atalyst (from 0.001 to 1 molar equivalent), the silane compound (1 equivalent), and the solvent are introduced into a Schlenk tube, which is then sealed with a J. Young tap. The concentration of amine and of silane compound in the reaction mixture is approximately 1M (concentration calculated on the basis of the volume of solvent introduced). The order in which the reactants are introduced is not important. [0127] The Schlenk tube is subsequently placed under CO2 pressure (f rom 1 to 3 bar) by means of a vacuum ramp, and then is heated to a temperature of between 25 and 100°C. until full conversion of the amine (from 5 minutes to 72 hours of reaction). [0128] When the reaction is at an end, the volatile compo unds are removed under reduced pressure and the reaction mixture is purified by chromatography on silica gel. The use of THF as an eluent allows any silyl-containing byproducts to be recovered (mixture of siloxanes and silanols). In a secon d phase, ethyl acetate is used as the eluent, in order to recover the formamide compound. The ethyl acetate present in the solution thus collected is then removed under reduced pressure, to give the analytically pure formamide compound.
